Silver chloride
Material
AgCl
Silver chloride (AgCl) has a transmission range between 0.42 µm and 24.5 µm and is used for optics in the far IR range. Silver chloride is available as a white crystalline solid, which can turn dark grey after prolonged exposure to light, which is due to the splitting into metallic silver and chlorine. Silver chloride is practically insoluble in water, but can be dissolved in aqueous solutions of ammonia, potassium cyanide and sodium thiocyanate. It is corrosive when combined with metals. For the combination with metallic elements, an intermediate layer of Tefon or silver must be provided. AgCl is often used in sample cell windows of gases and liquids for IR and FTIR spectroscopy instead of optics made of potassium chloride (KCL), as these would be attacked by aqueous samples.
Properties
Spectral properties
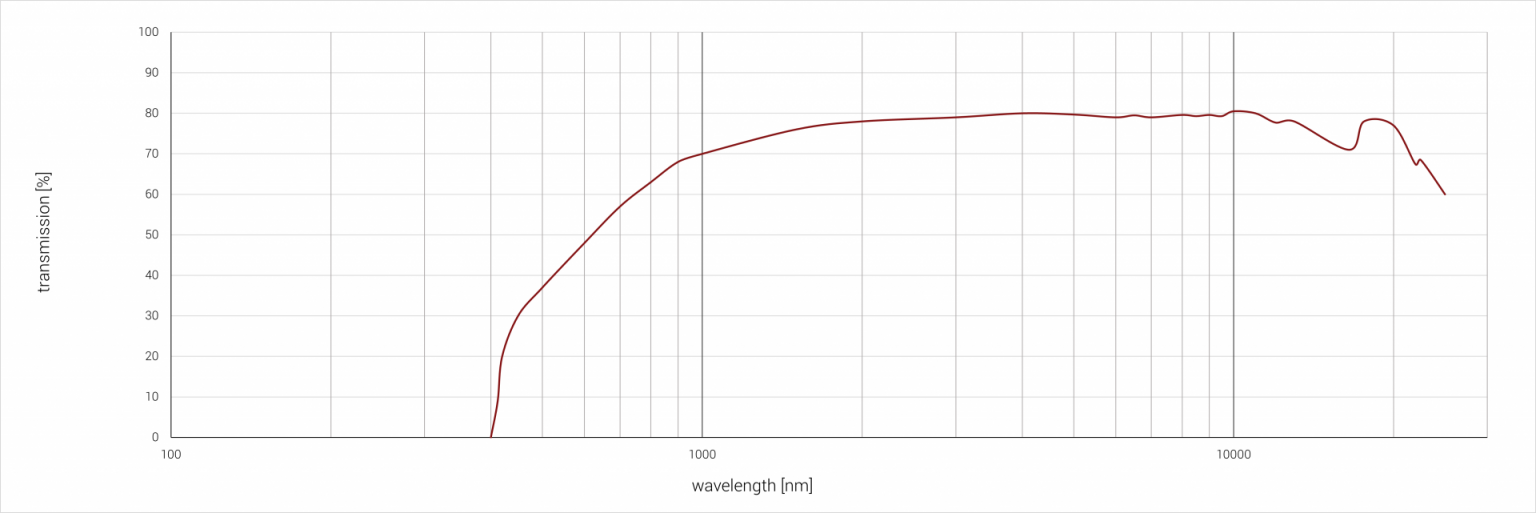
Sample thickness: 2 mm

